Our exploration of outer space offers us a rare opportunity to rethink our idea of "earth." Today, many years after man first viewed the earth from the moon, we still scurry around pursuing our daily lives and tending to believe that the earth is a very large, and unchanging sphere of rock, dirt, vegetation, and water, made exactly to our specifications. The view from outer space tells us a different story. Suddenly, we realize that there is a blanket of gases around us that is not infinite, and perhaps should not be taken for granted. Standing on the ground, buffeted by wind and rain and snow, we think we’re looking up into a seemingly vast and endless mass of gas. In truth, our earth’s air fills a very small container, a container defined not by plastic, glass, or rubber, but by the earth’s gravitational pull.
 The
earth’s gravitational gas container doesn’t even reach as high
as the moon, 238, 855 miles away.http://nssdc.gsfc.nasa.gov/photo_gallery/photogallery-earthmoon.html
(This image was taken by the Clementine moon-mapping satellite as it came
over the north lunar pole at the completion of mapping orbit 102 on March
13, 1994)
The
earth’s gravitational gas container doesn’t even reach as high
as the moon, 238, 855 miles away.http://nssdc.gsfc.nasa.gov/photo_gallery/photogallery-earthmoon.html
(This image was taken by the Clementine moon-mapping satellite as it came
over the north lunar pole at the completion of mapping orbit 102 on March
13, 1994)At a mere 365 miles straight up, an easy day’s car ride, the earth’s atmosphere stops. Above that, all the way to the moon and beyond, there is only occasional molecules and lonely specks of space debris. Until you reach Venus or Mars, our neighbors, there is nothing— just an immense vacuum.
Our gas-enveloped earth becomes even more interesting if we imagine what happens during a return trip from the moon. The first leg of the journey, some 238,500 miles, we encounter nothing! Three hundred and sixty five miles above the earth, we run into our first gas molecules. They don’t make much of an impression. At an altitude of 250 miles, where Space Station Alpha orbits, there are a few more gas molecules, but, again, we still can’t play outside without special suits and oxygen supplies.
As we approach the earth, the blanket of air above us grows denser, and the pressure of the gas molecules being pulled down towards the earth grows greater and greater. Thirty miles above the earth is an important milestone. At this altitude, 99.9% of all the gases around the earth are still beneath us. The mountain climbers we observe high on Mt. Everest, 5.5 miles above sea level, are wearing oxygen masks.
Below Everest’s peak, 3, 4, and even 5 miles up, where private airplanes fly, oxygen must be added to the plane’s cabin so that the passengers can breathe. At about 1 to 2 miles, or 5,000 to 10,000 feet, the gas molecules in the air become dense enough to let us breathe comfortably. Finally, when we land we realize that the earth is embraced by what is actually a very thin, and, as it turns out, fragile blanket of gas.
 |
 |
 |
Human Respiration
We inhale the gases of the air. Our body’s chemistry and physical makeup uses these gases to keep us alive. This story begins when air is inhaled through the nose and mouth. The air moves down the throat, through the larynx and trachea, and into the bronchi. The two bronchial tubes enter the lungs where they branch into smaller tubes called bronchioles. The air follows this path and ends up in small air sacs found at the end of each bronchiole. The air sacs are called alveoli.
If there is enough air pressure, the oxygen will pass through the membranes of the alveoli into the tiny blood vessels, called capillaries. The oxygen attaches itself to the blood's hemoglobin, forming oxyhemoglobin and is carried throughout the body to the cells in our muscles, our organs, and our nervous system.
 |
Many human cells are tiny factories. They use oxygen to burn the carbohydrates, fats, and proteins that have been transported to them from the food that was processed in our digestive systems. These cellular processes are called oxidation and reduction. Together they produce the energy that keeps our bodies at 98.6 Fahrenheit and allows us to think clearly, study our homework, run, and chew, and swallow more food.
Meanwhile, the burning of oxygen within the cells produces carbon dioxide. Carbon dioxide is a waste product from the burning. The carbon dioxide enters the blood stream and moves back through the heart to the lungs. In the lungs it passes outwards through the alveolar membrane. We get rid of this poisonous gas when we breathe out—it’s one form of human "exhaust." The burning process in the cells also produces water vapor. Along with the carbon dioxide, we breathe out small amounts water vapor, which is why our breath will fog up a mirror on a cold morning.
The Space Station: A "Bubble" in Space
From what you have just read, you understand why it is so important to pay close attention to the atmospheric conditions in the space station? The astronauts' minds and bodies continue to work and process oxygen and fuel and produce carbon dioxide and water vapor, just as they did on earth. In the very, very thin atmosphere of space, the astronauts still require earth-like conditions to keep their minds and bodies healthy. They need the same amount of oxygen to breathe and the same amount of air pressure they had on earth to ensure the oxygen will pass through the lining of their lungs.
As they exhale the astronauts pollute the space station’s atmosphere. Work and exercise turn them into factories without environmental control systems. Consider the gases they produce. Carbon dioxide, trace elements and water vapor are either poisonous, as in the case of carbon dioxide, or, as in the case of too much water vapor, can cause damage if too much condensation forms on the space station’s sensitive electrical equipment.
The astronauts also produce methane, ammonia, urea, and other poisons and noxious gases that can really make a mess of the space station’s air. If allowed to accumulate, these natural human by-products may cause illness and equipment failure. The accumulation of carbon dioxide in the atmosphere can cause carbon dioxide poisoning (see table below).
| The Affects upon Human Beings of Carbon Dioxide as a Percentage of Total Air Pressure. | |
| At ppCO2 0.2508 mmHg / .03% | No side effects. |
| At ppCO2 0.456 mmHg / .055% | Air seems "stuffy." |
| At ppCO2 0.76 mmHg / .092% | (Critical Level) Some people may begin to experience shortness of breath, rapid pulse rate, headaches, hearing loss, hyperventilation, sweating, and fatigue. Astronauts are trained to recognize these symptoms. |
| At ppCO2 3.8 mmHg / .46% | Too much time spent in these conditions may be permanently dangerous to the astronauts' health, especially if the amount of oxygen decreases. |
| At ppCO2 11.4 mmHg / 1.38% | (Mission Danger Level) The astronauts suffer serious symptoms within an hour or two. These symptoms include nausea, dizziness, mental depression, physical convulsions, and problems seeing. |
| At ppCO2 22.8 mmHg / 2.75% | Immediate health symptoms. Loss of consciousness may occur. Increased concentration of gas may prove fatal. |
Sustaining an Earth-like Environment
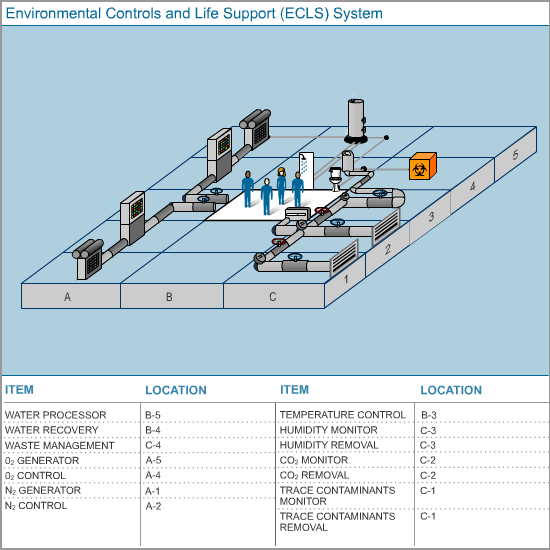
The space station’s scientists, engineers, and technicians designed the life support systems to create and maintain a clean, earth-like environment within the space station. Computer sensors take constant readings and signal the equipment designed to adjust and maintain the correct gas composition of the air. Nitrogen and oxygen stored in tanks is added to the atmosphere as needed. Carbon dioxide scrubbers are turned on and off as needed. De-humidifiers remove excess water vapor from the air.
Sensors are the eyes and ears of the space station's Environmental Controls and Life Support Systems (ECLS system). This system is an ingenious web of technology that supports the physical needs and presence of the astronauts. Maintaining a healthy atmosphere in the space station is a constant challenge. In case of technical problems, there are multiple back-up and safety devices on board. The atmosphere must be monitored constantly, because a change in the mixture of gases, in the pressure of the different gases, or of the total air pressure on board the space station could endanger the astronauts without them even knowing about it.