

The mark of a good solution for engineers is to find the correct balance between needs and available technology. In their quest for the perfect battery, engineers are looking for a way to deliver large amounts of electricity for long periods of time in a small package. Such a solution could truly keep the Energizer bunny® drumming forever. In this never-ending quest, engineers have experimented with lead, carbon, nickel, and a whole array of other exotic elements.
A battery, by definition, depends upon a reaction between different types of chemical elements to create a flow of free electrons. When the battery is connected to a device in a circuit, the electric current flows through the circuit providing electrical power to the device. A simple AA, AAA, C, or D Cell battery provides 1.5 volts of electrical current. In each case, the electricity is created by chemical reactions taking place inside the battery.
For many years lead has been used as a metal in batteries. However, lead is a very heavy material, and is too heavy to lift into space. More recently scientists have developed batteries using nickel (Ni). These batteries provide greater electrical output than the traditional lead-based materials. They are also lighter, provide more electricity per volume, last longer, and can be recharged by electricity from the PV arrays.
The space station is equipped with Nickel-Hydrogen battery assemblies. The batteries on the space station must have enough capacity to supply continuous power to the station while the PV arrays are in the shadow of the Earth.
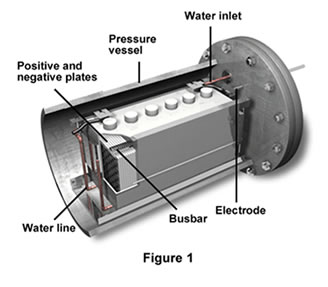 |
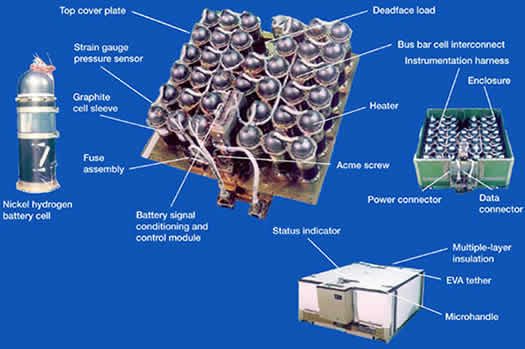
The illustration above shows a nickel-hydrogen battery that can produce
6-volts of current. The battery above is used on Earth. A different model
had to be designed for the weightlessness of space. The nickel-hydrogen
battery contains water (H2O). The hydrogen (H2) in the water molecules
undergoes a chemical reaction when it comes into contact with the nickel
(Ni) plates. This chemical reaction creates the energy that releases the
electrons. The electrons flow towards the negative end of the battery
and from there move into the electric cables that are linked to the space
station's electrical circuits.
Charging and Recharging
The space station orbits the Earth once every 90-95 minutes. About
two-thirds of the station's orbit is in direct sunlight. This part of
the orbit is called the "insolation period." (You might remember
this as "IN the SOL-ation", or "in the sun" period.)
One third of every orbit is in the Earth's shadow and is called the "eclipse
period." During the insolation period, the PV arrays power the space
station's equipment. In addition, some of the electrical power created
by the PV arrays is used to recharge the bank of nickel-hydrogen batteries.
While in eclipse, the batteries take over the job of supplying the station's
electrical power.
Using careful calculations, the scientists and engineers who designed the space station figured out how many nickel-hydrogen batteries had to be linked together to meet the station's electrical needs and still have enough electricity to spare in case anything should go wrong. They decided that only 35% of all the batteries' stored energy should be used up during the eclipse period. This leaves 65% of the batteries' electrical power for emergencies. Remember, during the insolation period the PV arrays take over again-- providing the electricity for the equipment in the space station, and recharging the batteries.
