

During the 1960s, the United States was competing with Russia in a great space race. The race spurred on both scientific invention and the scientific imagination. One of the most imaginative writers of that day was Gene Roddenberry, a former war pilot and policeman-turned-television scriptwriter. He was the man who envisioned Star Trek and the starship Enterprise. In this wonderful story, a multinational crew lives and works together, exploring the far reaches of the universe. In some ways, with the building of the International Space Station, the Enterprise has turned into reality. As the station orbits the earth with its international crew, it provides a platform to explore the frontier of space.
A fictional substance called dilithium (dye-li-thee-um) crystals powered the Enterprise between galaxies. With these crystals, Roddenberry provided one of the most important resources needed to sustain life in space: an endless supply of energy. According to the science behind the fiction, dilithium crystals created electricity from a reaction between matter and antimatter.
The space station’s dilithium crystal is the sun. As the station orbits from sunlight to shadow, from darkness back to light, the sun's rays generate the electrical power needed to sustain life on board the space station. While in the earth's shadow, nickel-hydrogen batteries provide power to the station.
Just like Roddenberry's Enterprise, the space station requires an uninterrupted flow of electricity for its life support systems, computers, telecommunications equipment, and experiments. Electricity is the station's lifeblood. Without electricity, the space station would be uninhabitable.
Solving the Energy Supply Problem in
a Vacuum
The sun spews enormous amounts of light-energy
out into the solar system. The earth receives only a small fraction of
this energy. Nevertheless, one minute's worth of solar energy shining
on the earth's atmosphere is greater than the energy consumed by the world
in a year. Put another way, the light shining upon one square meter of
the earth's outer atmosphere is enough energy to power a 1,350-watt hair
dryer endlessly. Effectively capturing and taming this immense reservoir
of energy and turning it into electricity has been the dream of scientists
and engineers for many years.
Turning Sunlight Directly into Electricity
The quest to transform sunlight directly
into electricity began nearly 170 years ago. In 1839, a French physicist
named Edmund Becquerel discovered that if a bright light is focused on
a metal surface, electrons jump from the atoms of a metal strip suspended
in a saltwater solution. He could not explain what was happening, but
he could measure an electrical current flowing between the metal strip
on which he had focused the light and another metal strip that was suspended
nearby in the water. Becquerel's discovery would later be known as the
photoelectric effect.
No progress was made with the photoelectric effect until several ideas came together between 1897 and 1905. In 1897, physicist J.J. Thompson provided the first evidence for the existence of electrons. Soon thereafter, a group of scientists were exploring the relationships between light, radioactivity, magnetism, and electricity. Max Planck, a German physicist, suggested that light behaves not only as waves, but also as small "packets" of energy called photons. In 1905, Einstein connected all of the earlier discoveries together. He said that the photon’s energy is transferred to an electron when it collides with an atom. In turn the electron releases a photon of energy in the form of light. This process is what Becquerel had witnessed years before.
In the 1950s, scientists at Bell Laboratories made a huge discovery. They found that the electrons in silicon atoms responded to the light from the sun in much the same way as the atoms in Becquerel's metal strips had responded. The space race motivated Bell Labs' scientists to find new ways to modify silicon crystals that would transform solar energy into the electrical energy needed to provide endless power for communications satellites.
Photovoltaic Cells
The space station receives much of its
energy from photovoltaic (PV) arrays. These arrays are results of the
most advanced research and experimentation in silicon crystals. Photons
of light from the sun collide with the silicon atoms and transmit energy
to the atoms’ electrons. The energized electrons jump from the silicon
atoms, leaving the atom ionized. The atom now has a positive electrical
charge. Impurities injected intentionally into the silicon crystal help
promote the flow of free electrons. The process of injecting impurities
into the silicon crystal is called "doping". The "impurities" are molecules
of the elements boron and phosphorous. The free electrons flow towards
the negative pole in the PV array. From there, they travel into the space
station’s electrical circuits.
Below is a diagram of how an earth-bound PV array
produces electricity.
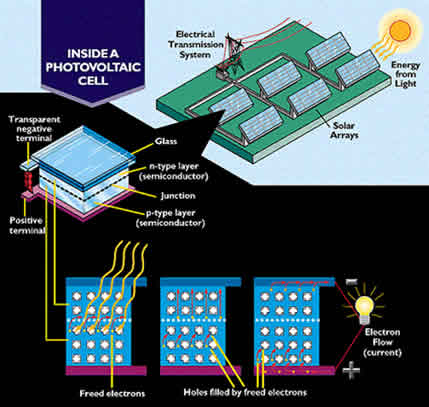 |
The boron atoms buried in the "p-type layer", which can be seen in the illustration above, keep the electrons flowing toward the n-type layer. As the electrons accumulate, they create an electromotive force. This force drives the electricity out along the copper wires leading to the space station and back to the PV array's positive terminal. This primary circuit on the space station is the power grid.
Football Fields of Solar Cells
Scientists designed the space station's
PV arrays by calculating how much electricity is required to run all of
the station's equipment. The PV arrays generate a total electromotive
force of 168 volts that passes through a transformer into the space station
at 124 volts. By comparison, houses in the United States have either a
120 or a 240-volt electrical service. The PV arrays continue to produce
this electromotive force while the space station is in sunlight.
In order to create a photovoltaic array, engineers first connect 200 PV cells together into a panel. Multiple panels are then joined, forming the array. The more panels used, the more electrical energy you can generate.
At completion, the station will have eight solar arrays arranged in several flexible wings that can be closed or opened from within the space station. Each of the arrays will measure about 107 feet (32 m) in length and 38 feet (11.5 m) wide. They will be connected to a center truss 310 feet (94 m) long. Each array will consist of many panels that are attached so they open and close like an accordion. When fully extended, the eight arrays will cover an area of 32,000 square feet, or about two-thirds the length of a football field. They are the largest deployable structures ever to be sent into space.
