|
Teacher Lesson Pages
During the 1960s the United States was competing with the Soviet Union in a great space race. The race brought on both
scientific invention and scientific imagination. One of the most imaginative writers of that day was Gene Roddenberry,
a former war pilot and policeman. He was the man who wrote Star Trek about the starship Enterprise. In this wonderful story,
a crew from many countries and many planets live and work together. They explore the far reaches of the universe.
In some ways, with the building of the International Space Station, the Enterprise has turned into reality. As the station
orbits Earth with its international crew, it provides a platform to explore the frontier of space.
In Star Trek, a fictional substance called dilithium (dye-li-thee-um) crystals power the Enterprise between galaxies. With
these crystals Roddenberry provided one of the most important resources needed to sustain life in spaceóan endless supply
of energy.
Our space stationís energy source is the sun. As the station orbits from sunlight to shadow, the sun's rays generate the
electrical power needed to sustain life on board the space station. When the station is in Earth's shadow, nickel-hydrogen
batteries provide power to the station.
Just like Roddenberry's Enterprise, the space station requires an uninterrupted flow of electricity for its life support
systems, computers, communications equipment, and experiments. Electricity is the station's lifeblood. Without electricity
the space station would be uninhabitable.
Solving the Energy Supply Problem in a Vacuum
The sun spews enormous amounts of light energy out into the solar system. Earth receives only a small fraction of this energy.
Nevertheless, one minute's worth of solar energy shining on Earth's atmosphere is greater than the energy consumed by the
world in a year. Effectively capturing this immense amount of energy and turning it into electricity has been the dream of
scientists and engineers for many years.
Turning Sunlight Directly into Electricity
The quest to transform sunlight directly into electricity began in 1839 when a French physicist named Edmund Becquerel
discovered that if a bright light is focused on a metal surface, electrons jump from the atoms of a metal strip suspended
in a saltwater solution. He could not explain what was happening, but he could measure an electrical current flowing between
the lighted metal strip and another metal strip nearby in the water. Becquerel's discovery would later be known as the
photoelectric effect.
No progress was made with the photoelectric effect until several ideas came together. In 1897 physicist J.J. Thompson provided
the first evidence for the existence of electrons. Soon thereafter, a group of scientists were exploring the relationships
between light, magnetism, and electricity. Max Planck, a German physicist, suggested that light behaves not only as waves, but
also as small "packets" of energy called photons. In 1905 Albert Einstein connected all of the earlier discoveries together. He
said that the photonís energy is transferred to an electron when it collides with an atom. In turn, the electron releases a
photon of energy in the form of light. This process is what Becquerel had witnessed years before.
In the 1950s scientists at Bell Laboratories made a huge discovery. They found that the electrons in silicon atoms responded
to the light from the sun in much the same way as the atoms in Becquerel's metal strips had responded. The space race motivated
Bell Labs' scientists to find new ways to modify silicon crystals that would transform solar energy into the electrical energy
needed to provide endless power for communications satellites.
 Photovoltaic Cells
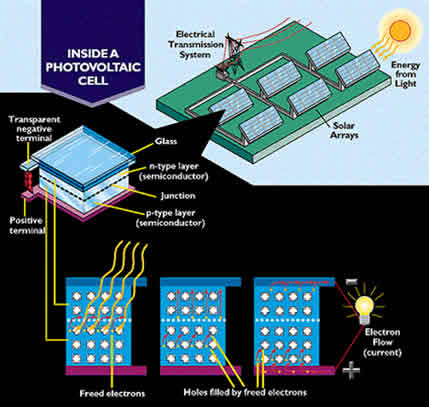
Photovoltaic Cells
The space station receives much of its energy from photovoltaic (PV) arrays. These arrays are results of the most advanced
research and experimentation in silicon crystals. Photons of light from the sun collide with the silicon atoms and transmit
energy to the atomsí electrons. The energized electrons jump from the silicon atoms. Free electrons flow toward the negative
pole in the PV array. From there they travel into the space stationís electrical circuits.
Football Fields of Solar Cells
Scientists designed the space station's solar arrays by calculating how much electricity is required to run all of the station's
equipment.
In order to create a photovoltaic array, engineers first connect 200 PV cells together into a panel. Multiple panels are then
joined, forming the array. The more panels used, the more electrical energy you can generate.
When it is completed, the station will have eight solar arrays arranged in several flexible wings that can be closed or opened.
Each of the arrays will measure about 107 feet (32 m) in length and 38 feet (11.5 m) wide. They will be connected to a center
truss 310 feet (94 m) long. Each array will consist of many panels that are attached so they open and close like an accordion.
When fully extended, the eight arrays will cover an area of 32,000 square feet, or about two-thirds the length of a football
field. They are the largest deployable structures ever to be sent into space.
Check for Understanding
Answer the following questions to see how much you know about the space stationís energy supply problem.
- What is the space stationís energy source?
The sun.
- How does the space station make the electricity needed to power all the stationís systems?
Photovoltaic arrays are made of solar panels wired together to collectively produce more electricity from sunlight.
Photons of light from the sun hit silicon atoms in solar panels and transit energy to electrons. The electrons jump
and flow toward the negative pole of the PV array. Electrons flowing over a wire is electricity.
|


