

The electromagnetic radiation produced by the tube or plasma in your television or by your microwave oven is friendly energy. This energy is working for you. If you are reading this article in class, friendly electromagnetic radiation in the form of visible light is being produced by the light bulb above you and the sun outside. Electromagnetic radiation in the form of X-rays or gamma rays, which can penetrate the cells of your body and cause the mutation of human DNA, can be your enemy. Harmful radiation may come from sources as varied as the sun, hydrogen bombs, the uranium in the soil of the Rocky Mountains, or even teeny amounts of radon in a basement.
Electromagnetic radiation is all around us. It moves at the speed of light. It cooks our food, enters our eyes, bathes our skin and heats us, penetrates our bodies and our cells, and provides plants with the energy to conduct photosynthesis. Some forms of radiation are easy to control. Some forms are far harder to control and can be very dangerous to human beings.
What is Electromagnetic Radiation?
Before you explore electromagnetism further, it’s
important that we avoid any confusion. Electromagnetic radiation is referred
to in a number of different ways. Here are a few terms and phrases that
you will encounter when reading about electromagnetic energy: electromagnetic
waves, ionizing radiation and non-ionizing radiation, light, and, sometimes,
simply radiation. All of these terms and phrases refer to electromagnetic
energy in one form or another.
Even though scientists had been studying light for ages, physicists really only began to identify different forms of electromagnetic radiation in the early part of the last century. A wave of energy traveling through space, through a vacuum, unsupported by any known medium such as air, was an idea which scientists themselves had to get used to. As hard as this was to fully accept, imagine the problems that arose in the rational minds of scientists when they began to discover that electromagnetic radiation existed in two different states at the same time. This was almost like saying water is both a gas and a liquid at the same time rather than two completely separate phases of matter.
The discovery of the dual nature of electromagnetism was one of the great achievements of the physical sciences during the twentieth century. At first scientists believed that electromagnetism existed only in the form of waves. As electromagnetism is emitted from any source, whether a simple heated metal pan, a flashlight, an X-ray machine, or the fusion or fission of two atomic nuclei, it moves through space in the form of a wave.
Electromagnetic waves were found to have two of the same characteristics as waves in the ocean; namely, length and frequency. The length of a wave is the distance between two consecutive wave troughs or peaks. Because all electromagnetic waves are moving at the same speed, the speed of light, not as many long waves would flow past a buoy in a given period of time as would short, choppy electromagnetic waves. For this reason, long waves are said to have a low frequency. Waves whose peaks are closely spaced together have a high frequency. Using water as an example, electromagnetism in the form of waves was easy to understand.
Imagine the surprise that scientists experienced when it was discovered that electromagnetic radiation also behaved like infinitesimally small energy packets or particles, called photons. When you think of minute particles you might think of a particle of dust or sand, certainly not of energy particles. (The same words used in different contexts can certainly cause confusion!) Sand and dust particles are solid, molecular substances. Photons have no dimensions, only energy. When it was discovered that electromagnetic radiation also behaved like a flow of particles of dimensionless energy was quite a surprise. In fact, the idea at first appeared to defy all common sense. However, countless experiments have now demonstrated that electromagnetism does behave as if it were a particle of energy. In some experiments, certain intensities of light are aimed at atoms in metallic substances and, electrons break loose from the atoms in the substance in a way that proves that light is in fact not simply a wave but is in fact also a stream of electron-sized particles of energy.
Where Can I Find Electromagnetic Radiation?
As you have already read, electromagnetic radiation
is everywhere! Consider the illustrations below. Electromagnetic energy,
in the form of waves, exists in a variety of wavelengths called a spectrum.
Notice that the waves at the gamma ray end of the spectrum are much shorter
(meaning their peaks are closer together) and, therefore, higher in frequency
than the waves at the radio-wave end of the spectrum.
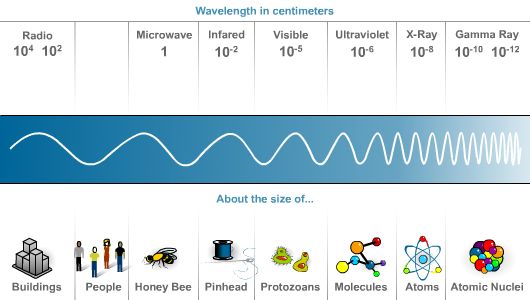 |
Because electromagnetic energy plays such an important role in the universe, and in our lives and that of the astronauts, here is a brief catalog of the electromagnetic spectrum and how humans put it to work:
• The longest waves, which are relatively low in energy and frequency, are commonly called radio and TV signals. These waves form a vital link in our communication networks.
• Microwaves are slightly higher in energy. This radiation is used in microwave ovens to prepare food. It is also closely monitored to signal extreme forms of solar weather.
• Infrared rays are used in short-distance wireless communication devices such as pagers, mobile phones, and notebook computers.
• The electromagnetic energies found in the center of the electromagnetic spectrum produce visible light. These waves are generated by such energy sources as the sun or a light bulb. They enter our eyes at the speed of light. Every second you spend looking at a red ball two hundred trillion waves of red light enter your eyes. The photons of energy stimulate the molecules in your eye's nerve endings and electrical impulses transmit the "idea" of light to your brain. Visible light photons also release the flow of electrons in the solar cells of the Photovoltaic arrays on the space station.
• One source of ultraviolet rays is the sun. To protect your skin from ultraviolet rays, you should shield your skin with sunscreen and your eyes with Polaroid sun glasses.
• X-rays are also produced by the sun, but they can also be artificially produced in X-ray machines used in hospitals.
• Gamma rays are produced during the thermonuclear reaction in the center of the sun called fusion. On earth we produce gamma rays in nuclear power plants and in atomic and hydrogen bombs.
This second illustration of the electromagnetic spectrum, seen below, includes the letter "A" to the left. "A" (with a small circle above it) stands for "angstrom." The angstrom is the metric unit used to describe the length of waves. One angstrom is equal to 10-10of one meter, or .000000001 meters.
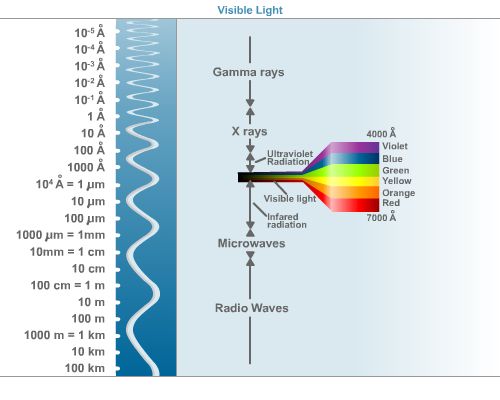 |
In the illustration above, notice that visible light has been expanded to a range of waves. Each segment of waves, or frequencies, in this range produces a different color. Radio waves also have a range of frequencies. This range is responsible for the different frequencies on your radio dial. Actually, all seven electromagnetic "families" on the spectrum cover a range of frequencies. Higher intensity X-rays are called "hard X-rays."
Where Does Electromagnetic Radiation
Come From?
All electromagnetic waves originate in atoms or
in the interaction of magnetic fields and electricity. In the case of
atoms, when the total energy of an atom is altered by an outside source,
the atom produces an electromagnetic photon. Each time scientists alter
the energy levels of electrons by slowing them down or speeding them up
the result is the production of some form of electromagnetic energy.
Although you do not say, "I can feel the electromagnetic radiation from that fire," that statement would be correct. During a fire, the atoms’ electron’s energy levels are excited as the heat energy spreads., As the molecules in the wood break down, the wood is chemically changed, more electrons are energized and energy is released as heat and light. As we can see from this example, energy, it turns out, can be transformed from one form to another. No energy is ever lost.
Under very highly controlled conditions, when nuclear scientists focus enough energy in the form of heat and pressure on the nucleus of one or more atoms, they can split the nucleus (fission) or cause one nucleus to fuse with another nucleus (fusion). The changes that take place in the atomic structures of the atoms involved produce extremely powerful electromagnetic energies. This is what happens in the core of the sun, but without the interference of nuclear scientists. Put simply, "messing" with atoms, whether by altering the energy levels of electrons or the nuclei of atoms, whether in the laboratory or in the sun, produces electromagnetic energy.
You might ask, "How much energy is produced?" In the case of fusion or fission, the energy (E) produced is equal to the mass (m) that is "lost" during the nuclear reaction, times the speed of light (c) squared. Do you recognize Einstein's famous equation? E=mc2! Practically speaking, when scientists focus enough energy on a softball-sized mass of uranium 235 atoms and start a chain reaction that eventually alters the atomic structure of each atom, they can produce a lot of very dangerous energy. This is known as a nuclear bomb. The amount of energy contained within one atom that is transformed from matter to energy would astound you.
Is All Radiation the Same, or Are There
Differences?
Essentially, all electromagnetic energy is the
same. It has the same properties, frequency, wavelength, and speed. Radiation
health workers, however, divide electromagnetic energy into two types,
non-ionizing and ionizing radiation.
Electromagnetic radiation, from radio waves and across the spectrum to visible light, is called non-ionizing radiation. Non-ionizing radiation, in its natural form, does not typically carry enough energy to alter the structure of molecules or atoms.
|
|
Ionizing Radiation
Electromagnetic waves with higher frequencies
than visible light can pack a wallop. These forms of energy are capable
of penetrating and altering an atomic structure. Ultraviolet rays can
alter the atomic structure of molecules in our skin, potentially causing
skin cancer. X-rays and gamma rays can penetrate our bodies and internal
organs. Some organs in our bodies, especially those that must constantly
produce new cells, are particularly susceptible to the affects of alteration
by ionizing radiation. The damage that can be caused by ionizing radiation
is all too tragically visible in the victims of nuclear accidents and
explosions.
Why Is an Understanding of Electromagnetic
Radiation Important for the Astronauts?
The space station orbits above the critical layers
of our atmosphere and passes through the magnetic field lines of the earth's
magnetosphere. The sun is a huge generator of both non-ionizing and ionizing
radiation. Scientists use a wide range of energy-sensitive technologies
to discover that the sun creates virtually every form of electromagnetic
radiation. During intense periods of solar weather ionizing radiation
in the form of X-rays, gamma rays, and from electrically charged, or radioactive
atomic particles is released into space and are of great concern to human
beings. Strategically positioned satellites monitor the sun’s activity.
Ionizing radiation from the sun is a constant hazard to the astronauts
on the space station.

